Antibiotic resistance is a growing public health concern, and according to the Centers for Disease Control and Prevention, drug-resistant infections account for 2 million infections and 23,000 deaths in the U.S. each year. The World Health Organization estimates that the threat of antimicrobial resistance will kill 50 million people per year by 2050. Superbugs such as C. difficile (which accounts for about 15,000 of those deaths), CRE, Neisseria Gonorrhoeae, MRSA, Streptococcus Pneumoniae, Malaria, and MDR are some of the top superbugs that commonly fall into this category. When antibiotics fail to work, there may be little to turn to in the medical world to prevent the downward spiral of a patient.
Stephanie Strathdee is an infectious disease epidemiologist who spent most of her career focusing on HIV prevention research. Since 2008, she has been associate dean of Global Health Institute overseeing the University of California’s San Diego campus. In November of 2015 while traveling in Egypt with her husband Tom Patterson a professor of psychiatry at the school of medicine, he developed stomach pain that worsened to where he was medevacked to a hospital in Frankfurt Germany. There, doctors determined that he had a life-threatening infection from a multidrug-resistant strain of Acinetobacter baumannii. Patterson was projectile-vomiting black bile and was unable to eat or drink anything. His family had to wear protective scrubs and gloves to come near him. With her husband’s condition worsening despite antibiotic therapy, she flew her husband Tom Patterson back home at the request of Dr. Robert Schooley who heads the division of infectious diseases at UC San Diego.
It was this turn of events and the failure of antibiotics that compelled Stephanie Strathdee to turn to a novel therapy to rescue him from death. She knew about a little- known therapeutic approach to treating infections that was discovered about one hundred years ago by a French-Canadian microbiologist named Felix d’Herelle and a British bacteriologist named Frederick Twort.
This therapy involved bacteriophages or phages for short, which are viruses that target and kill specific strains of bacteria. Modern researchers in the field of phage therapy such as Theron Hamilton of the US Naval Academy, Ryland Young of Texas A&M University, Carl Merril formerly of the NIH, and a phage clinic in Tbilisi Georgia were enlisted to help. Together, they developed bacteriophages some of which was developed from sewage water, that could be delivered intravenously and enabled Tom Patterson to awake from a coma with multiple organs failing within days of treatment. Patterson had relapses but after a few rounds of intravenous phage therapy, he fully recovered. In the two years since, physicians at UC San Diego Health have treated five other patients with phages for bacterial infections under emergency investigational new drug approval from the FDA. Encouraged by the broadly positive results observed in these patients, the UC San Diego Chancellor has announced a three year 1.2 million grant to help launch the first phage center in North America called the Center for Innovative Phage Applications and Therapeutics (IPATH).
Bacteriophages have been around since the dawn of the planet and comprise the single most abundant life form on the planet. There are an estimated ten million trillion trillion bacteriophages on earth. A single drop of seawater can contain millions. They exist in soil, water, and in excrement. While they are harmless to humans, phages are deadly to bacteria and prevent bacteria from overrunning the planet. Smaller than bacteria, bacteriophages have a very unusual appearance as you can see from this electron microscopic image. The difficulty in broadening phages as a treatment protocol is the need for a host where the bacteriophage can survive long enough to see if can
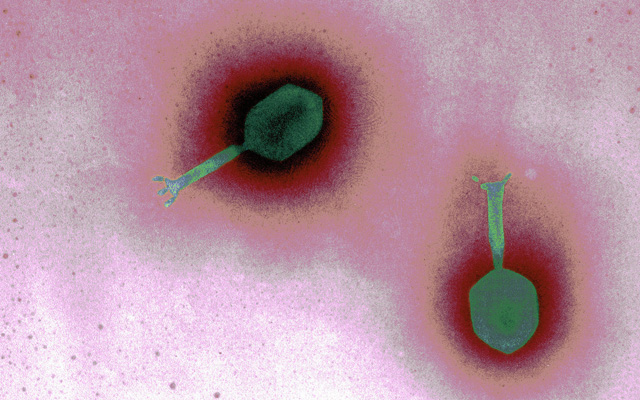
kill a specific bacterium. If the phages do in fact find a specific bacterium, they insert their DNA into the bacterium and then replicate themselves so that it causes the bacteria to burst open with multiples of themselves. The diagram below illustrates how this works.